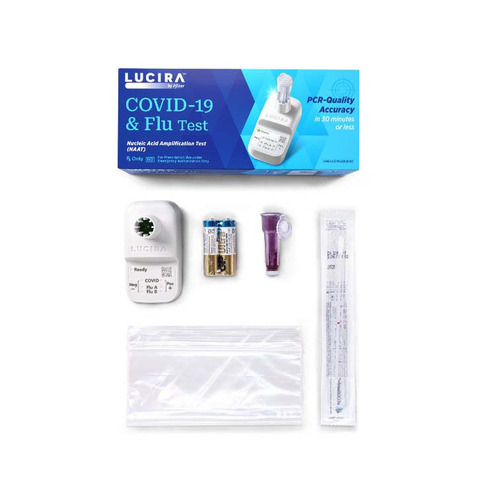
Pfizer Respiratory Test Kit Lucira COVID-19 / Flu A + B 1 Test
Lucira COVID-19 / Flu A + B Rapid Test Kit by Pfizer
Experience the convenience of the Lucira COVID-19 / Flu A + B Test Kit, an efficient solution for the rapid detection of respiratory infections. This test is designed for the qualitative detection of SARS-CoV-2, Influenza A, and Influenza B RNA using a simple anterior nasal swab sample.
Key Features
- Rapid Results: Obtain results within 30 minutes, making it ideal for quick decision-making.
- Instrument-Free: The single-use, molecular diagnostic test requires no calibration or ongoing maintenance.
- Emergency Use Authorization: Authorized for use under EUA by the FDA for specific laboratory settings.
- Point-of-Care (POC) Testing: Approved for use in patient care settings with a CLIA Certificate of Waiver, Compliance, or Accreditation.
- Comprehensive Detection: Simultaneously detect and differentiate between SARS-CoV-2, Influenza A, and Influenza B.
- Easy Implementation: No extensive training required, simplifying the process for healthcare providers.
The Lucira by Pfizer COVID-19 & Flu Test is a revolutionary tool in respiratory diagnostics, providing healthcare professionals with reliable and quick results. Positive results indicate the presence of the virus, although they do not exclude the possibility of bacterial infection or co-infection with other pathogens. Negative results for SARS-CoV-2 and Influenza B are presumptive and should be confirmed with an alternative authorized assay for accuracy.
Lucira, manufactured by Pfizer, is committed to providing innovative health solutions. This product is available for use under Emergency Use Authorization only and is intended for in vitro diagnostic use.
- Quantity : 1 Each
- Technology : RT-LAMP Amplification Technology
- UNSPSC Code : 41116144
- Time to Results : 30 Minute Results
- Test Type : Molecular Diagnostic
- Test Name : COVID-19 / Flu A + B
- Test Method : Nucleic Acid Amplification Test (NAAT)
- Test Kit Type : Rapid
- Test Format : Test Device Format
- Application : Respiratory Test Kit
- Specialty : Molecular
- Sample Type : Anterior Nasal Swab Sample
- Reading Type : Visual Read
- Purchase Program Type : Standard Purchase
- Product Dating : McKesson Acceptable Dating: we will ship = 60 days
- Number of Tests : 1 Test
- NDC Number : 00069970131
- Contents 1 : (1) Test Unit with Lyophilized Reagents and Electronic Readout, (1) Nasal Swab (Sterile Flocked Swab in Peel-Pouch), (1) Single Use Sample Vial, (2) AA Batteries for Test Unit, (1) Plastic Disposable Bag, Package Insert
Prescription Information
A valid medical prescription must be submitted to Betty Mills within seven (7) days for the ordered item(s), and shipping will be on hold until the prescription is received. If the prescription is not provided within this timeframe, the order may be canceled. This requirement does not apply to licensed care providers, training facilities, equivalent qualified professionals, or licensed wholesalers, who are exempt from this policy.
Prescriptions may be sent via:
19 South B Street Suite 8
San Mateo, CA 94401
You are entitled to a free consultation following the purchase of this item.
Product Documents
Clinical Laboratory Top Sellers
-
Vacutainer® Plus Venous Blood Collection Tube (MON 448214BX)
$53.72 Per Box
Customers also Viewed
This weeks Deals
-
Universal Folding Seat for 2-Button Walker, 2 EA/CS (MED G07872)
$52.52 Per Case